NAD/NADH测试盒
产品名称: NAD/NADH测试盒
英文名称: EnzyChrom™ NAD/NADH Assay Kit
产品编号: E2ND-048, E2ND-100
产品价格: 0
产品产地: 美国
品牌商标: BioAssay Systems(美国博世)
更新时间: null
使用范围: null
- 联系人 :
- 地址 : 成都市新都区泰兴普河工业园105号
- 邮编 :
- 所在区域 : 四川
- 电话 : 点击查看
- 传真 : 点击查看
- 邮箱 : support@basbioinc.com
|
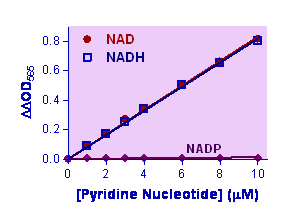
Description Pyridine nucleotides play an important role in metabolism and, thus, there is continual interest in monitoring their concentration levels. Quantitative determination of NAD+/NADH has applications in research pertaining to energy transformation and redox state of cells or tissue. Simple, direct and automation-ready procedures for measuring NAD+/NADH concentration are very desirable. BioAssay Systems EnzyChromTM NAD+/NADH assay kit is based on a lactate dehydrogenase cycling reaction, in which the formed NADH reduces a formazan (MTT) reagent. The intensity of the reduced product color, measured at 565 nm, is proportionate to the NAD+/NADH concentration in the sample. This assay is highly specific for NAD+/NADH and with minimal interference (<1%) by NADP+/NADPH. Our assay is a convenient method to measure NAD, NADH and their ratio.
Applications Direct Assays: NAD+/NADH concentrations and ratios in cell or tissue extracts.
Key features Sensitive and accurate. Detection limit 0.05 mM, linearity up to 10 mM NAD+/NADH in 96-well plate assay. Convenient. The procedure involves adding a single working reagent, and reading the optical density at time zero and 15 min at room temperature. No 37°C heater is required. High-throughput. Can be readily automated as a high-throughput 96-well plate assay for thousands of samples per day.
Kit Contents (100 tests in 96-well plates) Assay Buffer: 10 mL Lactate: 1.5 mL MTT Solution: 1.5 mL Enzyme A: 120 mLNAD Standard: 0.5 mL 1 mM Enzyme B: 120 mLNAD/NADH Extraction Buffers: each 12 mL
Storage conditions. Store all reagents at -20°C. Shelf life: 6 months after receipt.
Precautions: reagents are for research use only. Normal precautions for laboratory reagents should be exercised while using the reagents. Please refer to Material Safety Data Sheet for detailed information.
ProcedureS 1. Sample Preparation. For tissues weigh ~20 mg tissue for each sample, wash with cold PBS. For cell samples, wash cells with cold PBS and pellet ~105 cells for each sample. Homogenize samples (either tissue or cells) in a 1.5 mL Eppindorf tube with either 100 mL NAD extraction buffer for NAD determination or 100 mL NADH extraction buffer for NADH determination. Heat extracts at 60°C for 5 min and then add 20 mL Assay Buffer and 100 mL of the opposite extraction buffer to neutralize the extracts. Briefly vortex and spin the samples down at 14,000 rpm for 5 min. Use supernatant for NAD/NADH assays. Determination of both NAD and NADH concentrations requires extractions from two separate samples. 2. Calibration Curve. Prepare 500 mL 10 mM NAD Premix by mixing 5 mL 1 mM Standard and 495 mL distilled water. Dilute standard as follows.
|
|
Transfer 40 mL standards into wells of a clear flat-bottom 96-well plate. Samples. Add 40 mL sample per well in separate wells. 3. Reagent Preparation. For each well of reaction, prepare Working Reagent by mixing 60 mL Assay Buffer, 1 mL Enzyme A, 1 mL Enzyme B, 14 mL Lactate and 14 mL MTT. Fresh reconstitution is recommended. 4. Reaction. Add 80 mL Working Reagent per well quickly. Tap plate to mix briefly and thoroughly. 5. Read optical density (OD0) for time “zero” at 565 nm (520-600nm) and OD15 after a 15-min incubation at room temperature. 6. Calculation. Subtract OD0 from OD15 for the standard and sample wells. Use the DOD values to determine sample NAD/NADH concentration from the standard curve. Note: If the sample DOD values are higher than the DOD value for the 10 mM standard, dilute sample in distilled water and repeat this assay. Multiply the results by the dilution factor.
Materials Required, but not providedPipetting (multi-channel) devices. Clear-bottom 96-well plates (e.g. Corning Costar) and plate reader.
General Considerations 1. At these concentrations, the standard curves for NAD and NADH are identical. Since NADH in solution is unstable, we provide only NAD as the standard. 2. This assay is based on an enzyme-catalyzed kinetic reaction. Addition of Working Reagent should be quick and mixing should be brief but thorough. Use of multi-channel pipettor is recommended. 3. The following substances interfere and should be avoided in sample preparation. EDTA (>0.5 mM), ascorbic acid, SDS (>0.2%), sodium azide, NP-40 (>1%) and Tween-20 (>1%).
|